At the heart of 2-BBB’s CNS drug delivery platform lays the G-Technology®, a proprietary CNS targeted liposome formulation. Its attributes for existing and novel compounds are enhanced and localized CNS exposure, resulting in an improved therapeutic index. And its specific attributes for existing compounds are less unknowns and higher predictability, anticipated to result in lower development cost and lower failure risk as compared to novel compounds.
In an in vivo brain delivery study, the unbound brain-to-plasma concentration ratio (Kp,uu) increased ~10x by the G-Technology® versus control liposomes. In a computer model based on these results, it was estimated that the G-Technology® increased the therapeutic index ~100x for standard chemotherapy applied to brain cancers.
The central nervous system (CNS) is the part of the nervous system consisting of the brain and spinal cord. Along with many we consider the retina and the optic nerve as parts of the CNS as well. Our brain and eyes are not only shielded from outside impacts by the skull but also have protection from the inside by the perhaps lesser known blood-brain barrier and blood-retina barrier respectively.
Blood-brain barrier
The blood-brain barrier between the blood circulation and the brain acts as the biological equivalent of a computer firewall: it selectively allows nutrients and oxygen into the brain, while keeping out harmful components.
The blood-brain barrier is not fixed; it is in fact a dynamic barrier that is controlled by intracellular and intercellular signaling events among endothelial cells, astrocytes and neurons in the blood-brain barrier, as well as by other cells that are in contact with the barrier.
This function results from a combination of:
- a physical barrier – specialized connections (tight junctions) between cells reduce flux through the intercellular cleft or via the paracellular pathway;
- a transport barrier – specific transport mechanisms mediate solute flux; and
- a metabolic barrier – enzymes metabolize molecules in transit.
Physically, the blood-brain barrier is located in the endothelial cells of the cerebral capillaries. This capillary bed has impressive dimensions: the total length of capillaries in the human brain is approximately 600 km and has a surface area of about 20 m2. This means that almost every single neuron is perfused by its own capillary.
Although it is crucial in protecting the brain from toxic agents, the blood-brain barrier also blocks entry of most drugs into the brain, with more than 95% of drugs never reaching the brain in therapeutically relevant concentrations. This poses major difficulties for successful brain drug development.
Several approaches for direct drug delivery to the brain are currently under investigation by others, including direct single or continuous injections into the brain, cerebrospinal fluid or intranasal delivery. Most of these approaches have major disadvantages such as being too local, short lasting, highly invasive or, most importantly, not safe enough.
In contrast, the vascular route is a very promising approach for drug delivery to the brain as it allows for a widespread diffusion of the infused drug throughout the entire brain due to the large surface area of the human blood-brain barrier. Roughly, two concepts have been described in the literature to actively enhance drug delivery from the blood into the brain: temporary disruption of the blood-brain barrier or the use of endogenous transporters. Several methods are being developed by others based on disrupting the blood-brain barrier by osmotic imbalance or vasoactive compounds. These methods all carry the risk of temporarily or permanently damaging both blood-brain barrier and neurons due to unwanted blood components entering the brain. Therefore, physiological strategies to use endogenous transport mechanisms are preferable and have a large potential because of the inherent safety aspects of the targeted transport mechanism. This benefit is dependent on the endogenous function of the transporter and its endogenous ligand(s) not being impacted by the technology.
2-BBB leverages the G-Technology® to develop novel therapies involving an endogenous uptake mechanism, without interfering with its function.
Blood-retina barrier
The retina is protected by the blood–retinal barrier (BRB), which by engulfing all retinal blood vessels maintains retinal homeostasis and shields from, for example, blood-borne toxins or infectious agents. The BRB consists of two different barriers: (i) the outer BRB, which is formed by endothelial cells lining the choroidal vasculature (fenestrated blood vessels) and tight-junction-coupled retinal pigment epithelial (RPE) cells; and (ii) the inner BRB, which is formed by endothelial cells (non-fenestrated vessels) in conjunction with pericytes, astrocytes and Müller glial cells. The inner BRB is analogous to the blood-brain barrier (BBB), perhaps with the exception of Müller glia. The Müller glial cells could be of particular importance for the BRB because they form the outer and inner limiting membranes, which provide additional shielding of the neuroretina towards the RPE cells and the vitreous humor, respectively. Although the BRB is essential for the protection and viability of the retina, it can also prevent access of therapeutic compounds and, to date, relatively few studies have addressed this problem in a comprehensive way. As for the BBB, the G-Technology® could play an important role to improve the delivery of compounds to the eye.
Origin and value of the G-Technology®
The G-Technology® was developed by the Industrial Technology Research Institute (ITRI) in Hsin-Chu, Taiwan R.O.C. ITRI’s drug delivery department of Dr. Maggie Lu filed a series of patents describing glutathione-mediated drug delivery to the brain. Due to its pioneering work in the blood-brain barrier field, 2-BBB soon recognized the superior translational value of the technology. The well-known components of the formulation and the endogenous ligand would offer significant reductions in risk, time and cost of clinical trials and manufacturing. In a field where clinical technologies are sparse, the G-Technology® was most suitable to 2-BBB’s mission in transforming the lives of patients affected with brain diseases faster and more successful. 2-BBB has obtained the exclusive worldwide rights to commercialize these patents for the targeted delivery of drugs to the CNS.
Combining drugs with well-known, safe components to develop a novel medicine
The G-Technology® enables sustained delivery of systemically administered therapeutics while safely enhancing their brain and eye delivery. It carries therapeutics in small vesicles, so-called liposomes, thereby protecting the body from side effects caused by peak drug concentrations in the blood.
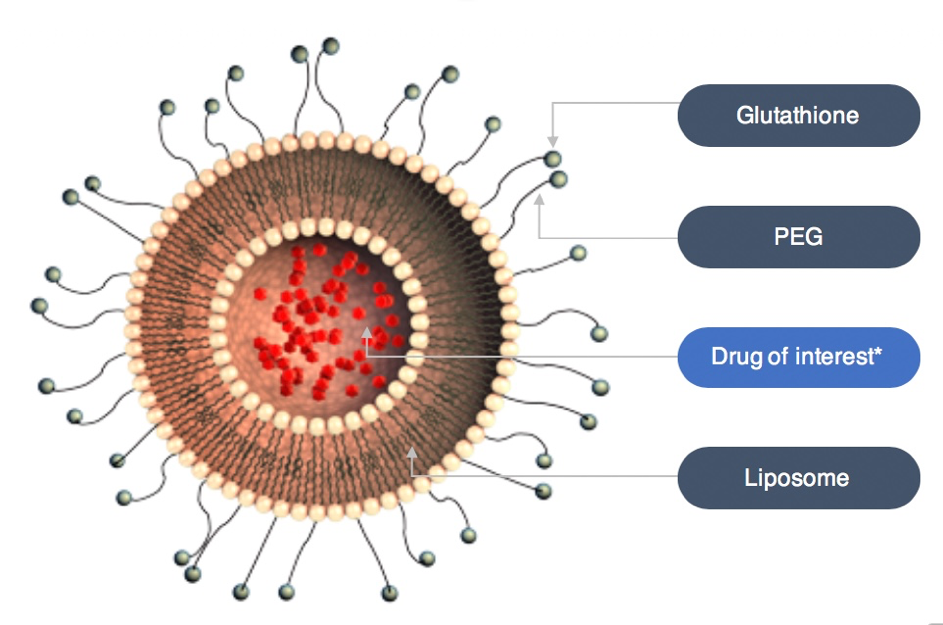
*Marketed or in development
Polyethylene glycol (PEG) is attached to the liposomes to ensure a prolonged circulation time in the blood stream. Glutathione (GSH) is conjugated to the PEG molecules to safely enhance delivery of the therapeutics across the blood-brain barrier and the blood-retina barrier.
An in vivo brain delivery versus controls study resulted in observed concentration-time profiles for unbound MTX in brain interstitial fluid and blood following intravenous infusion of free MTX, PEG liposomal MTX, and GSH-PEG liposomal MTX whereby Kp,uu increased ~10x (from 0.09 ±0.05 to 0.82 ±0.59). Inherently, the optimal brain-targeting effect is dependent on the encapsulated compound and the liposomal formulation that is combined with GSH. (European Journal of Pharmaceutics and Biopharmaceutics, June 2019)
An in silico brain delivery study underscored that liposomal formulation combined with GSH prevents therapeutic drug from being eliminated prior to reaching target tissue and simultaneously minimizes off-target toxicities, whereby the therapeutic index increased ~100x (from 1.7 to 159). (Journal of Pharmaceutical Sciences, October 2019)
